In many industries, especially in Pharma and Biotechnology, there is a global work environment with different people, from different countries, who are involved in joint projects and who manage a large volume of documents.
There are thousands of R&D records made in the process of drug production such as: multiple data collection points, transmission of records, many service providers involved, various control points.
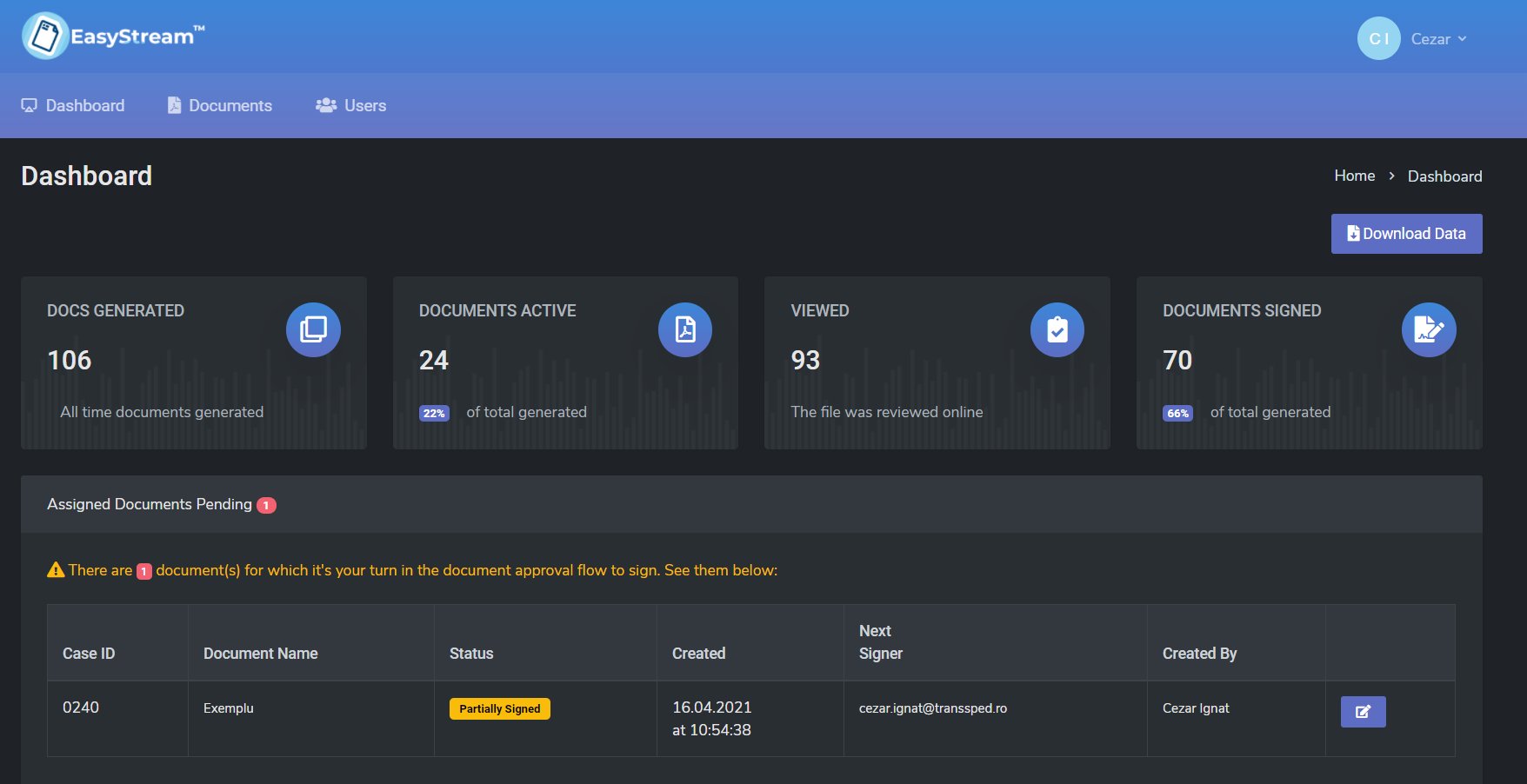
EasyStream simplifies the process of signing and approving documents, the system of chain-linked signing and approval makes signing documents easy and fast, from any device (smartphone, tablet or laptop).
TSP offers you an end-to-end solution to manage and monitor these activities.
In Pharma & Biotechnology we find complex processes: R&D centers, medical institutions, medical files, electronic evaluation of clinical results, pharmacovigilance forms, data management, clinical trial reporting, regulatory agencies, laboratory files, approvals and authorizations, product batch management, sales and distribution.
With the EasyStream solution from TSP you can manage all these processes in a single application, with transparency and traceability of flows, as well as a more efficient and optimized activity.
The signature made with qualified TSP certificates is non-repudiable and has the same legal value as the handwritten signature, compliant with European Regulation 910/2014 (eIDAS), ETSI and NIST standards, FDA ESG, FDA CFR part 11 and recognized by the Government of the United States of America.
Food and Drug Administration (US): 21 CFR part 11 (Allow the industry to use electronic records and signatures alternatively to paper records and hand-written signatures) https://www.fda.gov/industry/about-esg/esg-appendix-c-digital-certificates#4
European Medicines Agency (EU): eIDAS Regulation (on electronic identification and trust services for electronic transactions in the internal market) https://webgate.ec.europa.eu/tl-browser/#/tl/RO
US Federal Bridge PKI: https://playbooks.idmanagement.gov/fpki/tools/fpkigraph/
SAFE Identity: https://makeidentitysafe.com/get-safe-certified-credentials/

TSP solutions meet GxP requirements.
Signer identification is achieved through the TSP certified video identification solution, credentials are unique to each user, the qualified digital certificate uniquely binds the signature to the signer, includes two authentication factors and security measures to ensure that only the user can apply the signature. By using the signature together with the time stamp, the date and time of signing the document is certified and its integrity is ensured.

The final document is automatically archived in the long-term electronic archive solution, according to the legal provisions, guaranteeing the validity and preservation of the legal value of the document.
The documents are securely archived and can be available anytime, easily and quickly.
